Atoms are composed of three types of sub-atomic particles. Complete the following table to give their names, masses and charges.
| Name of Particle | Mass | Charge |
| Li | 6.941 | 3 |
| Mg | 24.3 | 12 |
| Cr | 51.996 | 24 |
2. Define the terms:
Atomic Number
“The atomic number of the chemical element is the number of protons in the nucleus of an atom of the element. It is the charge number of the nucleus since neutrons carry no net electrical charge. The atomic number determines the identity of an element and many of its chemical properties. The modern periodic table is ordered by increasing atomic number”(“Development of the Atomic Theory,” n.d.).
Mass number
“The atomic number of hydrogen is 1; the atomic number of carbon is 6, and the atomic number of silver is 47, Any atom with 47 protons is an atom of silver. Varying its number of neutrons changes its isotopes while changing the numbers of electrons makes it an ion”(“Atomic Mass – Chemistry LibreTexts,” n.d.).
3. Fill in the spaces in the following table
| Atom | Number of protons | Number of neutrons | Number of electrons |
| 27Al | 13 | 14 | 13 |
| P | 15 | 16 | 15 |
| Cl | 17 | 18 | 17 |
| Zn | 30 | 35 | 30 |
| 57Co | 27 | 32 | 27 |
| Zn | 27 | 32 | 27 |
| 131I | 53 | 74 | 53 |
| 135? | 55 | 80 | 55 |
Atomic masses are measured relative to the atomic mass unit. Define the atomic mass unit.
“An atomic mass unit (written as AMU or amu) is termed as exactly 1/12th of the mass of an atom of carbon-12. The carbon-12 or (C-12) atom has six neutrons and six protons in the nucleus of the atom. In others terms, one AMU is the average of the proton rest mass and the neutron rest mass. This is approximately 1.67377 x 10 -27 kilogram (kg), or 1.67377 x 10 -24 gram (g). The mass of an atom in AMU is roughly equal to the sum of the number of protons and neutrons in the nucleus”(“Chemical elements of the periodic table sorted by Atomic Mass,” n.d.).
The Atomic Mass Unit (AMU) is being used to state the relative masses of and thus differentiate it amongst numerous isotopes of any elements. Therefore, for instance, uranium-235 (U-235) has an atomic mass unit of about 235, whereas uranium-238 (U-238) is somewhat further huge. This alteration outcome from the point that U-238, a very abundant naturally existing isotope of the uranium element, has three further neutrons as compared to that of U-235, an isotope that is being used in atomic bombs and nuclear reactors.
Define the term ‘Relative Atomic Mass.
“The Relative Atomic mass of an element is the mass of an average atom of that element (taking into account its different isotopes and their relative proportions) compare with the mass of an atom of carbon-12”(“Relative Atomic Mass : GCSE Chemistry,” n.d.)
6. Complete the spaces in the following table:
| Symbol | Atomic No. | Mass No. | No. Protons | No. Neutrons | No. Electrons |
| Ne | 10 | 21 | 10 | 10 | 10 |
| Cl | 17 | 35 | 17 | 18 | 17 |
| Ne | 10 | 21 | 10 | 10 | 10 |
| Cl | 17 | 37 | 17 | 20 | 17 |
| Na | 11 | 22 | 11 | 12 | 11 |
| Co | 27 | 59 | 27 | 32 | 27 |
| Cr | 24 | 50 | 24 | 28 | 24 |
| Mn | 25 | 53 | 24 | 29 | 24 |
| Cr | 24 | 50 | 24 | 28 | 24 |
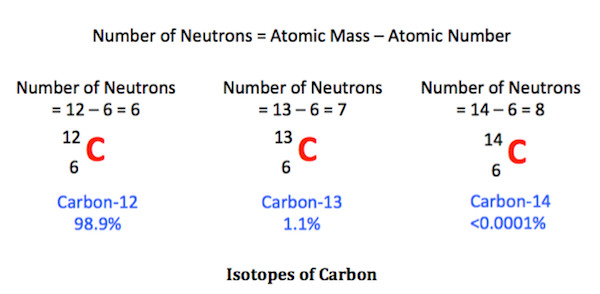
Define the term ‘Isotope
Isotopes are those atoms that have a similar sum of protons. However, they have different amounts of neutrons present inside an atom. In other words, they have dissimilar atomic mass but the same atomic number. Isotopes are the numerous forms of a single component.
There are a total of 275 isotopes out of 81 stable compounds. There are around 800 radioactive isotopes discovered till now. Several of them are synthetic, while some are natural. Each substance on the periodic table has many isotopic forms.
Identify any sets of isotopes in the above table, giving their symbols and mass numbers.
20Ne with an atomic mass of 19.9924356
21Ne with an atomic mass of 20.9938428
22Ne with an atomic mass of 21.9913831
Which quantity in the above table determines the identity of an atom, i.e., which element it is?
The quantity that determines the identification of an element is the atomic number, while atomic mass helps to identify the isotope of an atom.
Chlorine has two isotopes with mass numbers 35 and 37. On the Periodic Table, it is shown with a Relative Atomic Mass of 35.5. Use this as an example to explain the difference between mass number and relative atomic mass.
There are two main isotopes of the Chlorine atom. Around 75% of the Chlorine-atoms have a relative atomic mass of 35, and 25% have a relative atomic mass of 37.
Hence in a sample of Chlorine, the average relative atomic mass would be:
(75%×35)+(25%×37)=35.5
11. Naturally occurring gallium, atomic number 31, consists of a mixture of two isotopes with the abundance shown in brackets.
69Ga (60.2%) 71Ga (39.8%)
What is the relative atomic mass of naturally occurring gallium?
The relative atomic mass of naturally occurring gallium is 69.723 u ± 0.001 u
An artificial isotope of gallium has 41 neutrons. What is its mass number?
The mass number of the artificial isotope of gallium which has 41 neutrons, will be 71
12. Magnesium, atomic number 12, has three isotopes containing 12, 13, and 14 neutrons. The abundances of the isotopes are 78.60%, 10.11%, and 11.29%. Calculate:
The mass numbers of the isotopes
The mass number of the isotopes of magnesium is 54.93
b) The RAM of natural magnesium.
RAM of magnesium = 24/ {(1/12)*12} = 24
13. Write down the full electron configuration of the following atoms:
He: 1s2aa
C: 1s22s22p2.
Al: 1s22s22p63s23p1
V: 1s22s22p63s23p63d34s2
Mn : 1s22s22p63s23p63d54s2
14. Write down, using the ‘electron-in-box’ representation, the electron configurations of the following atoms:
Li: 1s22s1.
N: 1s22s22p63s23p63d104s24p64d105s25p5
O: 1s22s22p4.
Ne: 1s22s22p6
Cl: 1s22s22p63s23p5
Ni: 1s22s22p63s23p64s23d8
15. Write down the abbreviated electron configurations for:
B: [He] 2s2 2p1
N: [He] 2s2 2p3
Si: [Ne] 3s2 3p2
K: [Ar] 4s1
16. On a separate sheet, give a summary (600 words) of what you have learned during this assignment about the structure of the atom. Include annotated diagrams in your description and include the following
a) A brief history of atomic theory, including JJ Thomson, Dalton, N Bohr, E Schrodinger, and other famous scientists. Use diagrams to illustrate the changes in the structure of the atom.
b) Describe the main components of the atom and detail what you know about each of them.
c) What are isotopes? What are the uses of isotopes?
d) What is the significance of the electron?
e) What are the methods used for describing the energy of the electron? What does the notification 2p4 mean?
J.J. Thomson
J.J. Thomson’s has done experiments with the help of cathode-ray tubes that have shown that all of the atoms of an element comprises of small negatively-charged sub-atomic units within the atom, and it is called the electrons.
JJ Thomson’s plum-pudding prototypical of an atom of any element has negative-charged-electrons which are embedded inside a positive-charged soup.
Rutherford’s experiment of gold-foil has revealed that an atom is frequently unfilled spaces with a dense, tiny, and a +ve charged nucleus in it.
Based on these outcomes, Rutherford has suggested the nuclear-based prototype of an atom.
Dalton’s Atomic Theory
- All of the elements are made up of undividable elements; these particles cannot be further divided and are called as atoms, which can neither be created nor be destroyed.
- Those atoms which have similar elements have the same physical properties and mass.
- All of the chemical reactions are involved in the re-organization of the atoms.
Ernest Rutherford
Further ground-breaking experimentation in the past of the atom has conducted by the scientist Ernest Rutherford, a doctor from New Zealand who has spent his life in Canada and England. In his renowned experiment on gold foil, Ernest Rutherford has fired a very thin beam of α (alpha) particles at the very thinner sheet of the pure gold.
Niels Bohr
The discovery of electrons inside the atom and the discovery of radioactive elements and radioactivity at the conclusion of the 19th era has led to several prototypes for the identification of the structures of an atom. In the year 1913, Scientist Niels Bohr presented the theory for the atoms of hydrogen, which is founded on the quantum theory that says that energy is transported only in several well-distinct numbers. Electrons must circulate in the nucleus, however, only in the arranged paths. When moving from one trajectory to the other orbit with a lesser energy level, a quantum of quantum is produced. The theory presented by Bohr can enlighten why atoms produce light in particular wavelengths.
Figure 1(“Development of the Atomic Theory,” n.d.)
b) Describe the main components of the atom and detail what you know about each of them.
Atoms are the smallest and fundamental elements of matter and the defined structure of any element. The word “atom” derives from the Greek word for indivisible, as it was assumed that atoms are the tiniest particles in the whole universe and an atom is non-divided able.
Protons are +ve charged elements that are found inside the nuclei of an atom. Electrons are very small as compared to neutrons and protons, around 1,800 times less than of neutrons or protons. Electrons have a relative mass of 0.0005439 (as compared with the mass of a neutron being 1) or about 9.109×10-31 kg.
The neutron is mostly used as to compare and find out the comparative mass of electrons and protons. The existence of the neutron was conceived by the scientist Rutherford in the year 1920 and was discovered by Chadwick in the year 1932. Neutrons were founded inside the atoms in the experiments in which when atoms were fired at the thinner sheet of beryllium. Sub-atomic elements with no charge were released – the neutron.
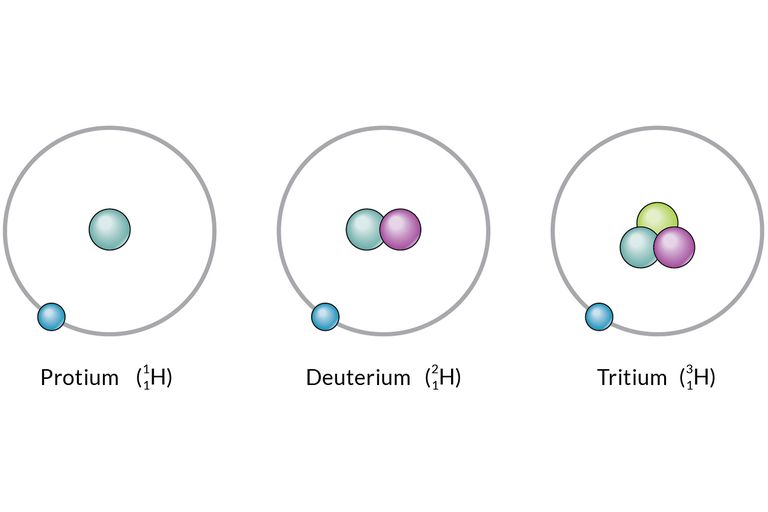
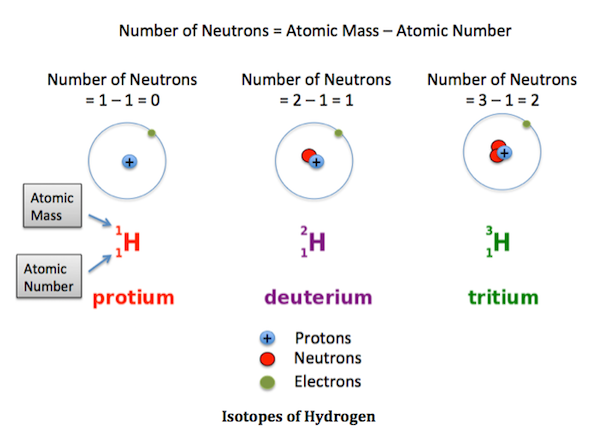
What are isotopes? What are the uses of isotopes?
Figure 2(“What Are Isotopes?” n.d.)
Isotopes are those atoms that have a similar sum of protons. However, they have different amounts of neutrons present inside an atom. In other words, they have dissimilar atomic mass but the same atomic number. Isotopes are the numerous forms of a single component.
There are a total of 275 isotopes out of 81 stable compounds. There are around 800 radioactive isotopes discovered now; several of them are synthetic, while some are natural. Each substance on the periodic table has many isotopic forms.
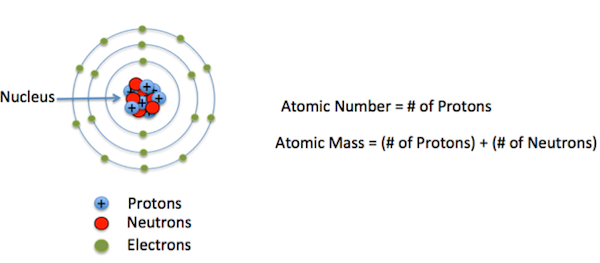
Figure 3(“What Are Isotopes?” n.d.) |
Isotopes of Hydrogen
|
|
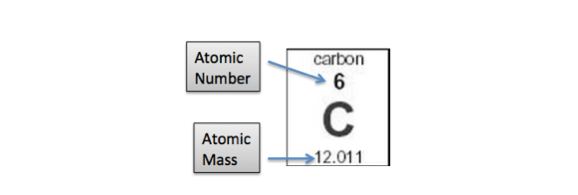
Figure 6(“What Are Isotopes?” n.d.) |
Uses of Isotopes
Isotopes are used in several ways. Some ways are listed below
Nuclear Magnetic Resonance Spectroscopy
Mass Spectrometry
Isotopic Labelling
What is the significance of the electron?
Electrons are the smallest as compared to that neutrons or protons, around 1,800 times lesser as compared to that of neutrons or protons. Electrons have a relative mass of 0.0005439 (as compared with the mass of a neutron being 1) or about 9.109×10-31 kg. An electron configuration of the atom is the orbital description of the position of the electrons in an atom. With the Use of the principles of physics and electronic configuration, the chemists could forecast the properties of the atom, for example, boiling point, conductivity, and stability.
What are the methods used for describing the energy of the electron? What does the notification 2p4 mean?
Two methods are used to describe the energy of the electron
Absorption of Photons
An electron of an element could absorb the lighter photon to reach the higher-energy state. However, the wavelength of the photon of light is the explicit wave length from every atom. Every atom, when placed in the spectroscope, creates many combinations of colors. These elements could only accept and produce the photon of light of definite wavelengths. If the wavelength has excessive or very little energy for that element, it would not be acknowledged. When the electron is in the excited state configuration, for it to come back down to its lower state, it produces a similar colored frequency of a photon to release the energy.
Collisions
When the elements hit, the electrons could be engaged from the lower state of energy towards the higher energy states. This happens as specific kinetic energy amongst the two striking atoms is moved towards the electron. Incoming the very fast impacts, the electron might be knocked free from its parent atom. This is also known as collision ionization. These electrons are then capable of being engrossed by the other atoms. Ionic bonds, which are created when the electrons are moved from one element to the other element, happen in this fashion.