Abstract
The purpose of this experiment is to study, evaluate and observe the variating behavior of 1-Butanol’s Entropy and Enthalpy of Vaporization based on variating temperature and pressure. For the observation of this variating thermodynamic behavior of 1-Butanol, the experiment was setup. The experiment was conducted thrice to ensure that stable results were observed. The purpose of this experiment is to evaluate the behavior of 1-Butanol in terms of enthalpy and entropy of vaporization when different levels of temperature and pressure are applied. For this purpose, three trials of experiments were conducted. The setup of the experiment was made using the Trevac-mechanical assembly. The obtained results were recorded. Later these results were graphically marked, and the behavior of 1-Butanol was compared with the theoretical linear equation for the verification of the results. This confirmed that the 1-Butanol presents a linear expansion when temperature and pressure are gradually increased. The behavior of 1-Butanol was in compliance with the Clausius-clapeyron-equation.
Introduction
According to the principles of thermodynamics, a volume comprises of immaculate sub-position in which physical sizes change based on the temperature and pressure. Considering this principle, the purpose of this experiment is to analyze the physical properties of 1-Butanol. Between two unique stages, physical properties/states may change irregularly. Such a change from one stage to another is called a phase change and is constantly associated with some misfortune or pick-up of entropy. A review of phase states and variations of an immaculate material is envisioned in supposed stage charts which portray the conduct of substance in connection to physical properties such as pressure, temperature, or volume. In such graphs, one can see that there are not just purposes of equilibrium where two stages remain in equilibrium, yet some of the regression lines.
Like this, the immaculate substances remain in equilibrium in the two stages at the ideal opportunity for serious variable sizes. In the equilibrium of the stages of liquid and gas, for instance, a similar measure of particles leaves the liquid as is caught by the liquid. By changing just, a single of the concentrated sizes and holding the other in similar esteem, the thermodynamic equilibrium of the substance is at last in only one stage alone, and all substance transforms into liquid or gas. Be that as it may, by changing the second size as well, it is conceivable to achieve an equilibrium once more, where the two stages are available.
A mixture of substances carries on considerably more entangled and complex physical properties than pure substances. For unadulterated substances, then again, the relationship between temperature and pressure obeys the Clausius-clapeyron-equation for the differential description of changes of the two sizes.
Problem Statement
Considering the above-described phenomenon, the purpose of this experiment is to briefly analyze, evaluate and observe the Entropy and Enthalpy of Vaporization of 1-butanol in Response to Changes in Temperature and Pressure.
Materials
- 1-Butanol
- Trevac-mechanical assembly
- Beaker
- Heating tubes
- Burner
- Pressure control beakers
- Thermometer
- Pressure-measuring device
Methods
To gauge the enthalpy of vaporization, 50 ml of 1-Butanol and bubbling stones were given into a vacuum pipe equipped with a warming mantle. The pressure was decreased until the point when the specimen was at reflux at room temperature, and the warmer was turned on. The pressure was then expanded to 400 mbar in ventures of 40 mbar and after that in ventures of 50 mbar to ordinary pressure. When the desired pressure was achieved the pressure was diminished in ventures of 50 mbar to 400 mbar, at that point in ventures of 40 mbar to 100 mbar. After each progression, the temperature was measure once the specimen was in equilibrium as it had common reflux.
Results
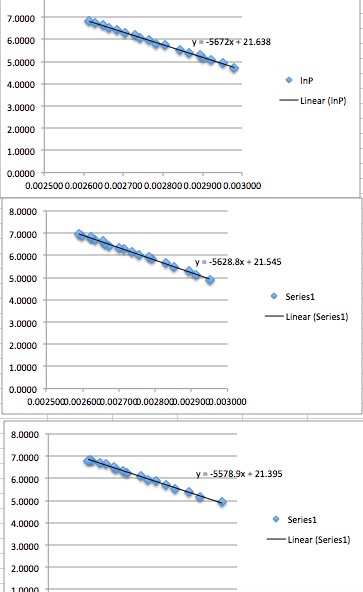
The purpose of this experiment was to evaluate the behavior of 1-Butanol in terms of enthalpy and entropy of vaporization when different levels of temperature and pressure is applied. For this purpose, three trials of experiments were conducted. The obtained results were recorded. Later these results were graphically marked and the behavior of 1-Butanol was compared with the theoretical linear equation for the verification of the results. This confirmed that the 1-Butanol presents a linear expansion when temperature and pressure are gradually increased. The behavior of 1-Butanol was in compliance with the Clausius-clapeyron-equation. The experiment was setup using Trevac-mechanical assembly.
The qualities found in the examination for vaporization enthalpy are a little too high, and the breaking point is marginally too low. By computing, the vaporization entropy rises the issue of which temperature to take. The utilized technique to take only the temperature normal of all deliberate temperature information focuses, and the vaporization enthalpy ascertained through the regression line prompts an outcome that lies in between the extreme theoretical values. With the figured increment of the relapse diagram of the two measured information lines for 1-butanol, one can find out the standard vaporization enthalpy of 1-Buthanol to ∆vH = 47.6 소 0.4 kJ mol−1.
The results of the experiment were theoretically verified and calculated using the following equations:
(ΔS = ΔH/T) and (LnP = -ΔH/R (1/T) + C)
The boiling point is given at 68.73 oC. For vaporization, entropy is discovered through the equation of two different estimations of 105.8 kJ K−1 (at 25oC) and 84.4 kJ/K (at 68.73oC). The qualities found in the experiment for vaporization enthalpy are too high, the boiling point is marginally too low. By figuring the vaporization, entropy rises the issue of which temperature to take. The utilized technique to take only the temperature normal of all deliberate temperature information points and the vaporization enthalpy ascertained through the regression line prompts an outcome that lies in the middle of the two writing esteems. Together with the normal temperature of the information points and equation, the vaporization entropy
Discussion
This outcome is extremely close to the writing estimation of 117.73 oC for the standard breaking point of 1-butanol. The writing estimation of the vaporization enthalpy is 52.35 kJ mol−1 at 25 oC, yet just 43.29 kJ mol−1 at TB = 117.73C. Therefore, the vaporization enthalpy is in no way, shape, or form steady in the inquired about territory of temperature. In an inverse to the exceptionally essential theoretic suspicion that it is steady comes about a distinction in the writing estimations of 20%. In any case, the computed vaporization enthalpy is by all accounts in the correct range between the two writing esteems, particularly with respect to the reality that the general normal temperature of the measured information focuses is 91.53 C. Actually, the examination is not sufficiently exact to manage these conditions of non-consistent vaporization enthalpy, and accordingly, the trial ought to be moved forward. The enthalpy of vaporization of 1-butanol was dictated by assessing the time-temperature profile given from the Trevac-mechanical assembly.
References
Wilhoit, R.C.; Chao, J.; Hall, K.R., Thermodynamic Properties of Key Organic Compounds in the Carbon Range C1 to C4. Part 1. Properties of Condensed Phases, J. Phys. Chem. Ref. Data, 1985, 14, 1.
Muñoz, Laura A.L.; Krähenbühl, M. Alvina, Isobaric Vapor Liquid Equilibrium (VLE) Data of the Systems n -Butanol + Butyric Acid and n -Butanol + Acetic Acid, J. Chem. Eng. Data, 2001, 46, 1, 120-124. [doi:10.1021/je000033u]
Wormald, C.J.; Fennell, D.P., Organometallics, 2000, 21, 3, 767-779. [doi:10.1023/A:1006648903706]
Dejoz, Ana; Cruz Burguet, M.; Munoz, Rosa; Sanchotello, Margarita, Isobaric Vapor-Liquid Equilibria of Tetrachloroethylene with 1-Butanol and 2-Butanol at 6 and 20 kPa, J. Chem. Eng. Data, 1995, 40, 1, 290-292. [doi:10.1021/je00017a064]