Introduction And Aims
Enzymes are biological globular proteins that catalyze the rate of biological reactions. They speed up the rate of many biological reactions, which otherwise would take ages to completion if in the absence of the enzymes. An enzyme is made of amino acids, hence protein in nature. The reactions catalyzed by the enzymes are reversible reactions Enzymes are never used up nor take part in the reaction as active metabolites but only speed up the time taken to reach the equilibrium of the equation. Enzymes have a high turnover point and are also specific in their reaction to the reaction they catalyze. Enzyme kinetics analysis the rates and factors affecting the rate of enzyme activities on the enzymes on the substrates. This can be investigated under varying conditions under which the reaction takes place.
Factor affecting the rates of the enzymatic reactions includes substrates and enzymes concentrations, temperature, Ph., inhibitors and the presence of modulators. Inorganic pyrophosphates, named EC 3.6.1.1, catalysis the hydrolysis of pyrophosphate (PPI) that proceeds as a bioproduct in many biochemical syntheses utilizing ATP as the source of energy. The enzyme splits a pyrophosphate molecule to form two phosphate ions in the presence of divalent metal cations and in a highly exergonic reaction .pyrophosphatese acts as a phosphoryl group donor. The enzyme pyrophosphatase has critical roles in the living organism. The enzyme plays an essential role in lipid metabolism, DNA metabolism, the formation of bones and neuron growth due to its exergonic nature; it is associated with various types of cancers and tumors, such as ovarian, lung and brain cancer.in fatty acid metabolism, a molecule ATP reacts with fatty acids to form acyl adenylate. The aim of this experiment is to study the enzyme kinetics of the enzyme pyrophosphates using pyrophosphates and acid molybdenum. The experiment analyzes the time taken to complete the reaction under different reaction conditions. The experiment will revolve around the effects of enzyme concentration, temperature and Ph.
Acidified ammonium molybdenum is used intensively in analytical chemistry to identify the presence of various compounds. It can be quantitively used to determine the presence of pyrophosphate in a color reaction. Pyrophosphate is highly reduced by blue molybdate acid and its compounds. In the presence of metal cations, the reaction will proceed with the formation of a blue color. The intensity of the blue color can be measured by absorbance. This will greatly aid in the aim of analyzing the pyrophosphate activity through the condition of enzyme concentrationpHH. And temperature.
Material And Method
In preparation for the standard curve, different phosphate concentration was used to react with a constant 2.5 ml of molybdate acid for ten minutes for the blue color to form. The intensity of the color was measured at 620 nm. The blank solution was prepared the same way as the reagent solution but lacked phosphate.
In investigating the effect of enzyme concentration, different amount of pyrophosphate enzyme was added. Magnesium chloride and triethanolamine buffer were used. On the effect of Ph, the experiment was carried out at varying ph. Condition Another factor was kept constant, but the buffer solution was not included.in investigating the temperature activity, the experiment was carried out at different temperature conditions, but previous factors were held constant.
Result Presentation
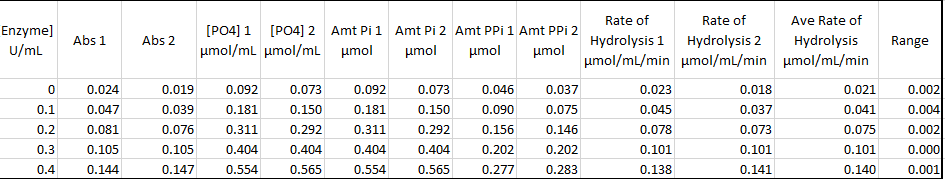
Table 1 Effect on enzyme concentration
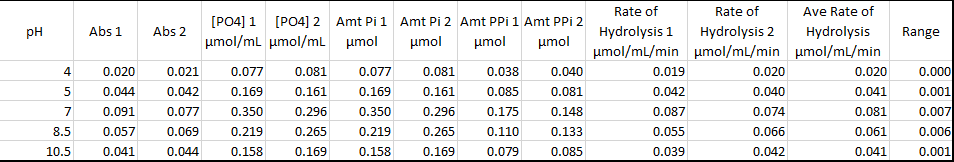
Table 2 Effect on Ph
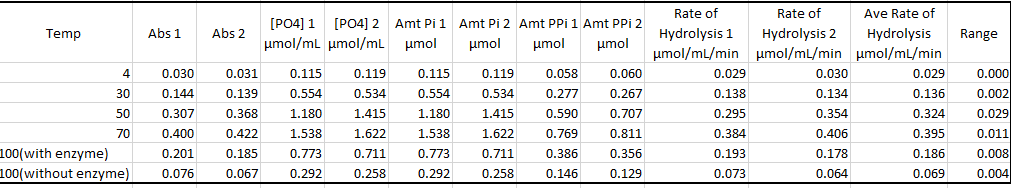
Table 3 Effect on temperature
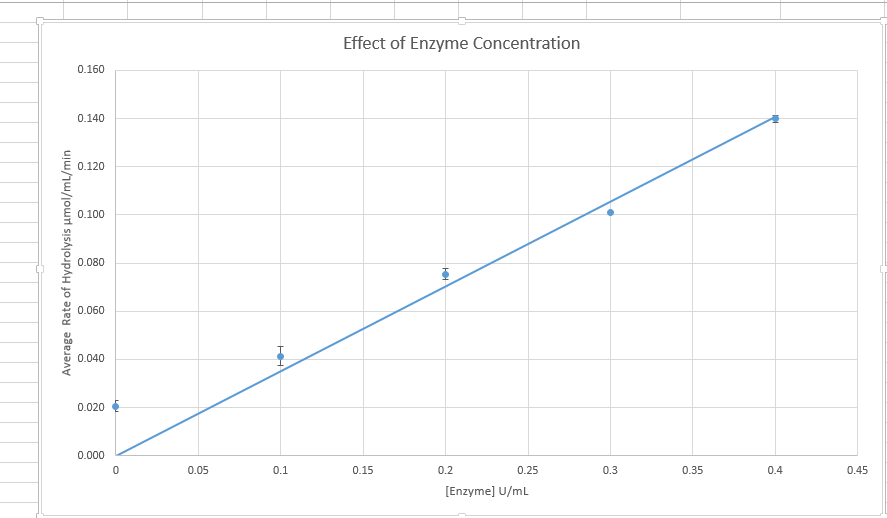
Figure 1 Graphs on the effect of enzyme concentration on enzyme activity
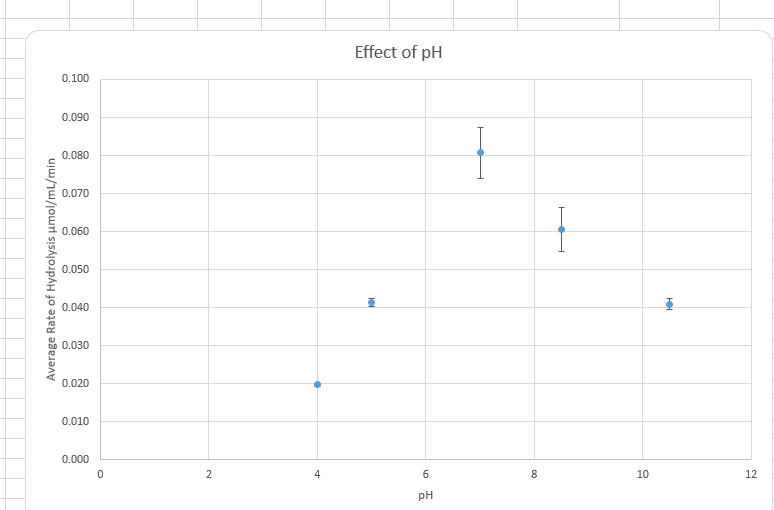
Figure 2 Graph on the effect of Ph on enzyme activity
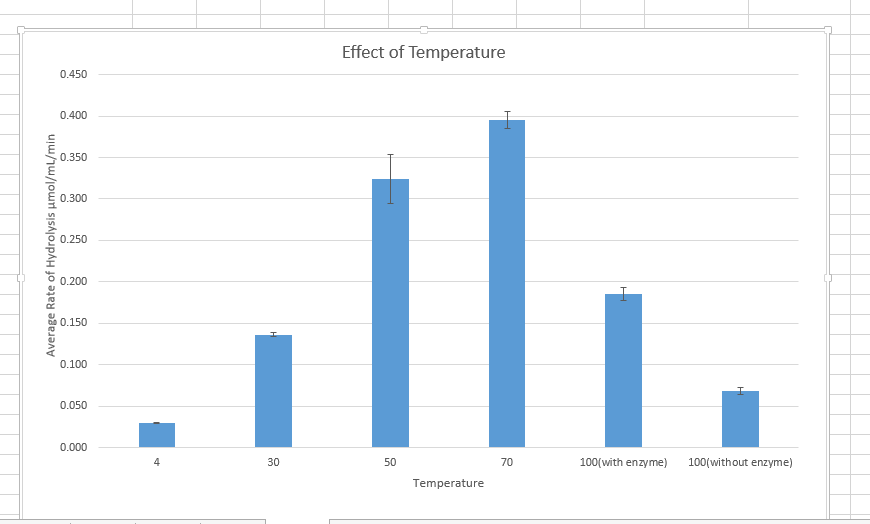
Figure 3 Effect of temperature on the effect of enzyme activity
Discussion
Enzyme activity is affected by the various factors of enzyme concentration, pH and temperature. The amount of enzyme concentration present affects the rate of a catalytic reaction with an increase of the reaction with the increasing substrate concentration and a lowering of the rate with a decrease of enzyme concentration. The enzyme catalyzes the reaction by binding to the substrate’s active site. Increasing the enzyme concentration increasing the chance s of enzyme combining with the substrate to form the product and the free enzymes. However, increasing the enzyme concentration suppressing the substrate concentration can cause e further increase, making the enzyme concentration the limiting factor in the reaction.
Temperature affects the rate of enzyme catalyzing the reaction by interfering with the enzyme. Temperature interferes with the stability of the bonds holding the enzymes together. Temperature also affects the kinetic energy of the reaction molecules, hence affecting the rate of collision and reaction. The different enzyme works best at certain optimum temperature conditions. An increase in temperature increases the rate of enzymatic reaction until an optimum point, after which the rate lowers. The decrease in temperature also reduces the rate of enzymatic reaction. Since the enzyme is a protein in nature, an increase in temperatures beyond the optimum points completely destroys the arrangement and organization of the enzyme structure, hence denaturing it. When temperatures also go below the optimum, it affects the structure of the enzyme rendering it inactive, however, an increase in temperature will activate the enzyme to normal working.
Ph is also a factor affecting the rate of enzymatic reaction. An increase in Ph increases the rate of enzymatic reaction until an optimum point is reached, after which a further increase in the Ph ionic strength slower the rate of chemical reaction. Each group of the enzyme have their optimum Ph working condition. Their enzyme works optimally in basic conditions, others in neutral Ph, and others in acidic conditions. Ph may affect both the structure and shape of the enzyme and substrate, causing the enzyme not to fit in the active site of the enzyme. This affects the rate of the reaction.
Data from the experiment correspond to the above theory lecture. According to Figure 1, enzyme concentration increased with the increase in enzyme concentration. In Figure 2 increase in p increased the enzymatic reaction until an optimum Ph of 7. According to Figure 3, an increase in temperature increased the rate of chemical reaction until the optimum of 70 degrees. After this enzyme becomes a denatured and further increase in temperature, the rate of reaction. However, there were some anomalies in the experimental data.
In collecting the anomalies, a more precise method of measurement can be used. Molybdenum compounds highly reduce pyrophosphate compared to the acid itself. The experiment can also be harnessed by the use of modulators, and the effect on substrate concentration and the effect of inhibitors can also be studied.
Conclusion
The experiment was a success in analyzing the effect of pyrophosphatase enzyme with acid molybdenum. The aim of the experiment was to analyze the activity of pyrophosphatase enzymes by investigating their activity on pyrophosphate. Factors of enzyme concentration, pH and temperature were investigated. This aim was achieved by the end of the experiment . Though slight human errors cannot be avoided, the observation of the experiments corresponded well with the expectations and the theory learned in class.
Appendix
A standard phosphate solution of 1mM /Ml had been prepared, and diluting this solution with different volumes of dilute water resulted in a different concentration of the phosphate solution. Different concentrations can be calculated with the formula
C1V1=C2V2
| Phosphate volume | Phosphate concentration |
| 0 | 0 |
| 0.20 | 0.2 |
| 0.40 | 0.4 |
| 0.60 | 0.6 |
| 0.80 | 0.8 |
| 1 | 1 |
| Abs 1 | Abs 2 | Average Abs | Range | |
| 0.000 | 0.000 | 0.000 | 0.000 | |
| 0.036 | 0.048 | 0.042 | 0.006 | |
| 0.114 | 0.104 | 0.109 | 0.005 | |
| 0.158 | 0.163 | 0.161 | 0.003 | |
| 0.209 | 0.207 | 0.208 | 0.001 | |
| 0.259 | 0.256 | 0.258 | 0.002 |
The absorbance measured showed different results for the different test tube reactions. The absorbance increased down the tubes for the standard reactions. Data deviated slightly but in a rage of (0-0.002).data was presented in graph and line of best fit formed a straight line, the line developed had a linear equation which can be presented in form of Y=MX+C. The linear equation developed can be presented as shown below
.Y=0.2602x, where y is the x-intercept while x is the gradient.
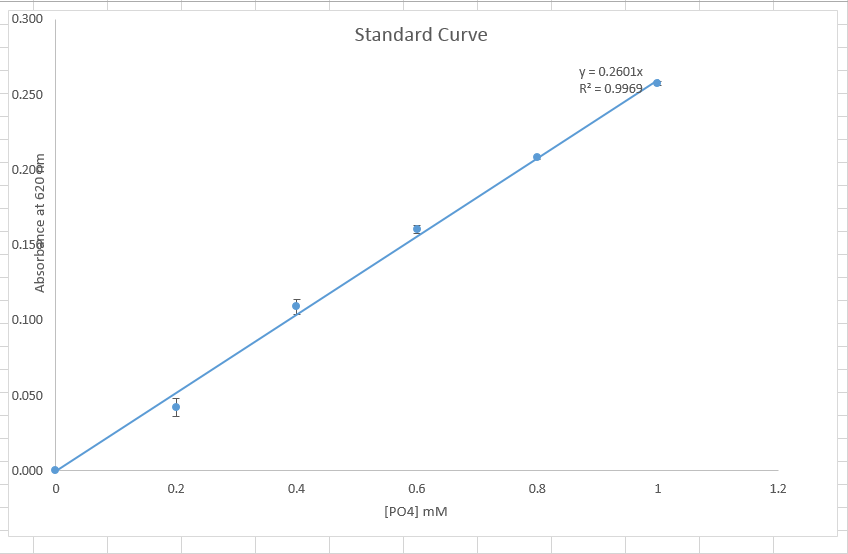
Figure a: standard curve.
Reference
Laidler, K.J. and Bunting, P.S., 1973. The chemical kinetics of enzyme action (Vol. 84). Oxford: Clarendon Press.
Tóth, J., Varga, B., Kovács, M., Málnási-Csizmadia, A., and Vértessy, B.G., 2007. Kinetic mechanism of human dUTPase, an essential nucleotide pyrophosphatase enzyme. Journal of Biological Chemistry, 282(46), pp.33572-33582.
VOLK, S.E., BAYKOV, A.A., DUZHENKO, V.S. and AVAEVA, S.M., 1982. Kinetic studies on the interactions of two forms of inorganic pyrophosphatase of heart mitochondria with physiological ligands. The FEBS Journal, 125(1), pp.215-220.
Cite This Work
To export a reference to this article please select a referencing stye below:
Academic Master Education Team is a group of academic editors and subject specialists responsible for producing structured, research-backed essays across multiple disciplines. Each article is developed following Academic Master’s Editorial Policy and supported by credible academic references. The team ensures clarity, citation accuracy, and adherence to ethical academic writing standards
Content reviewed under Academic Master Editorial Policy.
- This author does not have any more posts.




