Abstract:
Doxorubicin (DOX) is the most potent chemotherapeutic agent used for the treatment of various tumours. However, its active clinical use has been restricted due to hypothetically lethal cardiotoxicity. Increased reports have demonstrated that inhibition of anti-apoptotic factors, including BCL2 and BCL-XL, followed by increased pro-apoptotic factors, are critical mediators of DOX-induced cardiomyocyte loss. Melatonin, a hormone secreted by the penal gland, has shown some valuable protective effects against DOX-induced cardiotoxicity. However, the exact mechanism by which MEL exerts its cardioprotective effects remains unknown. H9C2 cardiomyocytes were cultured in Dulbecco’s Modified Eagle’s medium and treated with 0.5 μM of DOX alone, 0.5 μM of DOX + 1 μM of MEL and one μM of MEL alone for 24hrs. Following treatments, the expression BCL-2 and BCL-XL were assessed using a reverse transcriptase polymerase chain reaction to observe if MEL could attenuate DIC. The established H9C2 cardiomyocyte results are as follows: (i) DOX significantly reduced the expression of BCL-2, indicating apoptotic cell deaths, the value presented with 3-fold reduced expression mean SEM, n = 3, (p < 0.001). While MEL + DOX improved cell survival by partially blocking over 1-fold of DOX-induced BCL-2 inhibition, mean SEM, n = 3, (P<0.009) and MEL alone showed no gene alteration. (ii) DOX or MEL alone treatments unchanged the anti-apoptotic BCL-XL expression (p < 0.05). However, the MEL + DOX combination was significantly enhanced by 1.5-fold BXL-XL expression (P <0.03). The results suggest that strategies to activate the anti-apoptotic signalling molecules BCL-2 and BCL-XL could provide a novel advance to diminish DOX-mediated cardiotoxicity effects.
Keywords Doxorubicin; Melatonin; H9C2 cardiomyocyte; cardiotoxicity; apoptosis; BCL-2; BCL-XL
Abbreviation
- DOX Doxorubicin
- MEL Melatonin
- BCL-XL b-cell lymphoma 2-extra-large
- BCL-2 b-cell lymphoma – 2
- DIC Doxorubicin-induced cardiotoxicity
- ROS Reactive oxygen species
Introduction
The anthracycline doxorubicin originated from a modified sift of Streptomyces peucetiu and is one of the most potent antineoplastic drugs used for the treatment of various cancers (Giuseppe, 2016). The unique clinical use of this active anthracycline has been limited due to multiple adverse effects in notable cardiotoxicity. Patients affected by DOX-mediated cardiotoxicity may clinically present with irreversible and progressive early to late-onset chronic forms, ranging from left ventricular ejection fraction to arrhythmias and myocardial infarction resulting in cognitive heart failure (2 & 3). However, heart failure states may not be detected up to 20 years after completing DXR treatment, and the prognosis of those who develop DXR-induced HF remains indigent, with 50% fatality within two years. Underlining the urgent demand for cardioprotective adjuvant or novel therapeutics without disrupting the anti-cancerous effects.
The mechanism by which DXR induces cardiotoxicity remains complex. Inversely, the mechanisms involved in cardiotoxicity are separate from those mechanisms of anti-cancer therapeutic effects. The cardiotoxicity pathway involves inactivation of topoisomerase II β, TOP1MT, calcium homeostasis and reactive oxygen species (ROS) generations. TOP1MT and TOP II β inhibition trigger DNA injury-mediated apoptosis, mitochondrial genomes and cell transcription alterations. Particularly, TOP II β have shown a connection with PPARGC1A and PPARGC1B stimulated mitochondrial biogenesis decline and ATP-depletion. Similarly, calcium homeostasis dysregulation produces lipid peroxidation that subsequently diminishes the mitochondrial redox cycle, resulting in BCL-2/BAX ratio reduction and mPTP opening to discharge cytochrome – c, ultimately apoptosis (Ivanova et al., 2016) (Takemura et al., 2007).
DOX-induced Mitochondrial dysfunction
The ATP generator mitochondria are plentiful in heart tissue, forming about 35% of the cardiomyocyte volume. Mitochondrial impairment is a hallmark of DOX-mediated cardiotoxicity associated with ROS generation and mitochondrial bioenergetics distraction, causing insufficiency in the respiratory chain, oxidative phosphorylation and ATP production (2). The ROS containing hydroxyl radical, nitric oxide, superoxide and peroxynitrite is accountable for mediating DOX-induced cardiotoxicity through the enzymatic and non-enzymatic pathways. Subsequently, triggers DNA damage, protein carboxylation, lipid peroxidation, mitochondrial dysregulation, oxidative stress, and mPTP opening cytochrome c release, initiating apoptosis. Furthermore, in the enzymatic pathway, DOX interacts with cytochrome enzymes and mitochondrial respiratory chains, generating ROS and has a high affinity for binding to cardiolipin residing within the mitochondrial inner membranes. DOX has been shown to accumulate in the mitochondrial cardiolipin 100-fold higher than normal plasma concentrations, initiating cardiomyocyte cell deaths (3 &5).
Intrinsic apoptotic pathway
Cell death is crucial to DOX clinical limitation and mechanisms such as intrinsic apoptosis, extrinsic apoptosis, autophagic and necrosis cell death uses the mitochondria as a chief executioner throughout DXR-mediated cardiotoxicity involving disruption of the ETC, OXPHOS, and ATP production. The Intrinsic mitochondrial apoptosis pathway is instigated by internal stimuli, mainly by mitochondrial DNA impairment, intracellular ROS and excessive cytosolic calcium ions (4).
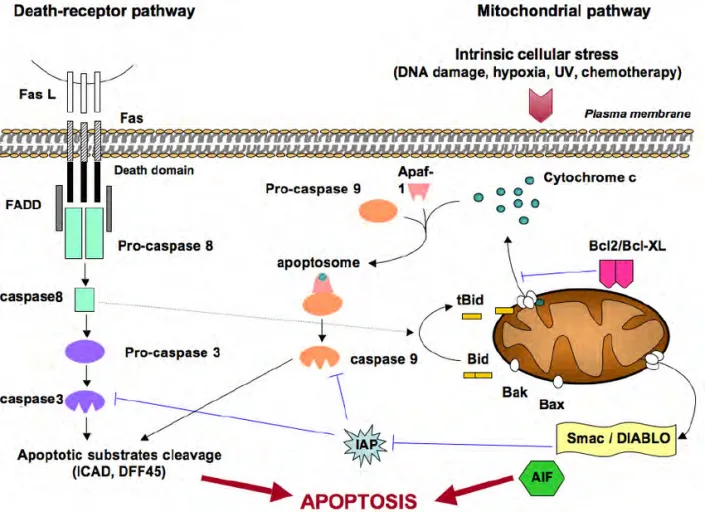
Figure 1: Extrinsic and intrinsic apoptotic initiating pathway: Anti-apoptotic proteins; Bcl-2, Bcl-W, Bcl-Xl. Intercellular ROD mediated upregulation of pro-apoptotic proteins; Bax, Bak, Bad, Bok, and cytochrome c release to apoptotic protease activating factor (APAF1), which activates caspase-9 and caspase-3 together with death-inducing signalling complex (DISC) stimulated caspase-8 and caspase-3 induced apoptosis.
The intrinsic pathway tightly controlled by the Bcl-2 protein family, including anti-apoptosis Bcl-2, Bcl-xL, pro-apoptosis Bax, Bak, and the BH3-only proteins, namely Bad, Bim, and Bid that increases apoptosis through blocking the antiapoptotic Bcl-2 or by stimulating the pro-apoptotic Bax / Bak. Accordingly, the anti-apoptotic controls apoptosis by inhibiting mitochondrial cytochrome c release, though pro-apoptotic proteins indeed release cytochrome c to activate caspase-9 to and executioner caspase-3, causing apoptotic cell deaths. Therefore, the ratios between the anti- and pro-apoptotic Bcl-2 family define whether apoptosis would occur or not. A study revealed, In H9c2 cells DOX-mediated a reduction in the expression of Bcl-2 and Bcl-xl activating PARP and caspase-3 resulting in apoptotic cell death (Yang et al., 2015). The overall clinical approach is to use antioxidants such as N-acetyl-5-methoxytryptamine Melatonin (MEL) to lessen the DXR-induced cardiotoxicity.
MEL attenuates DOX-induced cardiotoxicity
Melatonin is a pineal gland hormone; evidence from the last two decades documents its potential cardioprotective through anti-adrenergic, receptor-dependent/ independent actions. The expectant effect of MEL includes high potency on free radical scavenger, indirect antioxidant activity, anti-inflammation, prompting mitochondrial functioning, high solubility, low toxicity, cardioprotective and oncostatic roles. MLT can efficiently challenge events leading to mitochondrial damage by acting as a free radical scavenger and retaining the antioxidative potential to enhance the mitochondrial respiration complex chains, resulting in ATP production (Reiter et al., 2000).
Several studies have considered MEL as a possible novel cardio-therapeutic without interfering with DXR activity on antitumor, respectively, which explains its unique properties compared to other antioxidants. A recent study demonstrated MEL as more efficient than vitamin E by its capability to decrease the production of mitochondrial ROS, protect mitochondrial from H2O2-induced calcium overload and mPTP opening by significantly blocking the activation of caspase-3, RBA-1 cells from H2O2 -induced stress. Similarly, In H9C2 in vitro model, pre-treatment with MEL reduced hypoxia/reoxygenation (H/R) mediated apoptosis by activating ERK1 regulated IP3R and SERCA2a gene expression while significantly reversing H/R induced calcium overload in the cardiomyocytes.
Indeed, MLT has revealed a powerful anti-apoptotic influence to prevent ROS-induced apoptotic cardiomyocyte cell death. It has been shown to reduce oxidative stress events by blocking lipid peroxidation through scavenging more ROS, namely hydroxyl radicals, and reacts with ROS to form essential metabolite cyclic 3-hydroxy melatonin. That is more effective at scavenging free radical cascades, reducing oxidative stress to prevent mTOP, thereby inhibiting cellular apoptosis induced by oxidised cytochrome c release.
Even at the lower concentration, MEL revealed cardioprotection against myocardial ischemia-reperfusion injury by activating the protective downstream signalling pathways (Zhang et al., 2013). Additionally, DOX has been shown to accumulate in the mitochondrial cardiolipin 100-fold higher than normal plasma concentrations, initiating apoptotic cell deaths. Interestingly, MEL defends the mitochondria by inhibiting cardiolipin oxidation, which would otherwise stimulate the mPTP opening that leads to a downstream cascade of cell deaths (Paradies et al., 2010). Comparably, in cerebral ischemia, MEL directly blocked the mPTP opening using a dose-dependent approach and inhibited the apoptotic events. It has also shown antioxidative stress through the upregulation of SIRT1 and SIRT 3 associated with an increased anti-apoptotic Bcl-2, decreased Bax and caspase-3 (Andrabi et al., 2004) (Yu et al., 2014).
Indeed, MEL receptors may play an important role in its physiological significance since it functions primarily through high-affinity G proteins coupled receptors and possibly uses oligopeptide transports to mitochondria, performing higher antioxidant effect (Reiter et al. 2017). Nevertheless, the exact pathways in which MLT deliberates protection and the central cellular parameters that it influences continue to be clarified.
The approach consisted a semi-quantitative Reverse Transcriptase Polymerase Chain Reaction (RT-PCR) to analyses MEL and DOX effect on H9C2 cell through the BCL-2 intrinsic mitochondrial pathways. The H9C2 is driven from embryonic rate cardiomyocyte frequently used in numerous in vitro studies, particularly in cardiotoxicity analysis of chemotherapeutic drugs such as DOX and for novel cardioprotective treatments. The experiment included H9C2 tissue culture, treatments, RNA extraction, cDNA synthesis, Primer design through BLAST, gene expression was measured using PCR, and then PCR product was separated using agarose gel electrophoresis. Data were analysed using UV imaging, ImageJ documentation and Prism.
Hypothesis: The present investigation hypothesised that DOX would attenuate BCL-2 gene expression, and MEL could repress that effect to prevent cardiomyocytes against DOX-induced cardiotoxicity
Specific Aims: The study aimed to establish the H9c2 cell culture model to examine the cardio-protective of MEL on DOX-induced cardiotoxicity through BCL-2 pathways. Also, to design primer through BLAST for amplifying BCL-2 gene to run PCR efficiently. Image-J was used to semi-quantify BCL-2 gene expression to determine the anti-apoptotic level. Finally, compared the effect of DOX, DOX+MEL, and MEL with control genes in the H9C2 cell.
Materials
Dulbecco’s Modified Eagle’s Medium (DMEM; Catalog No. 30-2002, ATCC®, 2016), fetal bovine serum (FBS), Melatonin powder, Doxorubicin (Adriamycin®), Isopropanol and 75% ethanol were all purchased from Sigma-Aldrich. Kits pouched from Thermo Fisher Scientific: Trypsin-EDTA (0.25%) (Catalog No: 25200056), TM RiboLockTM RNase Inhibitor (40 U/µL) (Catalog No: EO0381), RevertAid H Minus M-MuLV Reverse Transcriptase (#K1631), RNase-free (#EN0521), TBE Buffer (Tris borate-EDTA) (10X) (Catalog No B52). TRIzolTM Reagent (Catalog No: 15596026), MyTaqTM HS Red Mix (Catalog N0:BIO-25047: Bioline Reagents Ltd).
Method
H9c2 cell line tissue culture
H9c2 cell lines originated from embryonic rat heart tissue and were purchased from the America Tissue Type Collection (Manassas, VA; catalogue # CRL-1446). The frozen cells in the vial were gently thawed for 2 minutes in a 37°C water bath and decontaminated by spraying with 70% ethanol. Then, to generate a complete growth medium, the contents were transferred into a centrifuge container containing DMEM supplemented with 10% FCS nutrients and 100 IU/ ml pencilling. Then, it was seeded into T-75 flasks (catalogue #430641) and incubated at 30°C in 5% CO2 for 24 hours. After achieving approximately 70% confluence, the cultures were split into 3X T-75 flasks. The culture medium was eliminated, and the cell layers were rinsed with 0.25% (w/v) Trypsin- 0.53 mM EDTA solution to abandon all traces of serum containing trypsin inhibitors. Then, 3.0 ml of Trypsin-EDTA solution was added into the flask to observe cell layer detachments under a microscope. Cells were aspirated by gently pipetting 8.0 ml of complete growth medium. When the suspension cells were 70% confluent, the cardiomyocytes were reseeded into 6 Well plates and stored at -80 °C. The medium was renewed and reseeded within 2 days, and the total passage number made was 20.
Drug dilution
DOX (Adriamycin® ) and MEL were bought from Sigma-Aldrich. DOX comes in 1mg and has a molecular mass of 544 g/mole. It was dissolved in 1.8 ml water to make a 1mm DOX stoke solution. Then, the 1mm (1000 μM) was diluted with 1:10 media to make the final working concentration 0.5 μM. Similarly, 2mg of MEL was weighed and mixed with 862 ul methanol to make a 10 mM (2.32 mg/ml) stock solution, then diluted 1:10 media to produce the final working concentration of 1uM. During this process the prepared MEL and DOX stock solutions were kept in Eppendorf tubes at -80°C, prior to experiment.
Treatments
Figure 4, H9C2 cell treated in 2x six-well plate Groups: A 1-3 no treatment, B 1-3 0.5 uM DOX treated for 24 hrs, C 1-3 cells pre-treated with media for 2hrs then with 0.5 uM DOX + 1 uM MEL for 24 hrs and group D 1-3 1 uM MEL only incubated for 24hrs.
Total RNA isolation
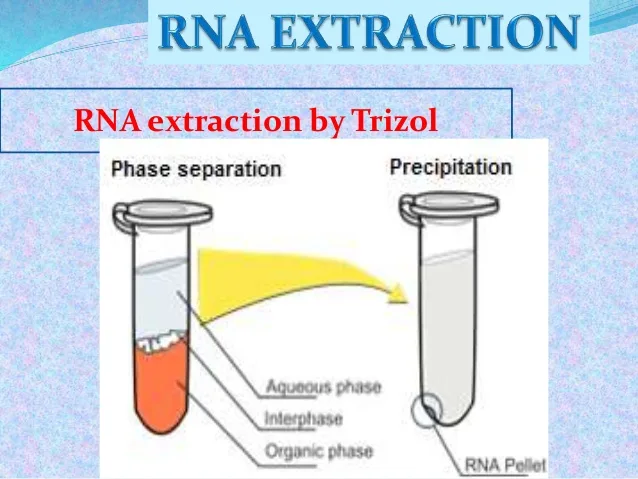
The gathering of total RNA isolation was achieved following the thermos fisher science instruction. In the separation phase, 0.5 ml isopropyl alcohol was used Per 1 ml of TRIZOL Reagent and vortex vigorously for 15 seconds, then incubated for 2 minutes at room temperature. The samples were centrifuged at 12,000 x g for 10 minutes at 8 0C, and a
colourless upper aqueous phase was formed where the RNA remained exclusively. The
Figure 5: The RNA separation phase by Trizol: organic phase, interphase, and aqueous phase participated in the formation of an RNA pellet at the bottom (16).
Precipitation: the RNA was precipitated from the aqueous phase by mixing with 0.5 ml of isopropyl alcohol per 1 ml of TRIZOL initially used in the homogenisation phase. Then, samples were centrifuged at 12,000 × g at 4°C for 10 minutes, and the whole RNA precipitate was visible as a white gel-like pellet at the bottom of the tube.
RNA Wash: The supernatant was removed thoroughly, and the RNA pellet was washed with 75% ethanol, adding 1 ml of 75% ethanol per 1 ml of TRIZOL to resuspend the pellet. Then, the sample was vortexed and centrifuged at 7500 × g at 4°C for 20 minutes, and a micropipette from the RNA pellet removed the supernatant. The washing procedure was repeated, and samples were kept at 50°C for 2 minutes to evaporate the remaining ethanol entirely.
Solubilize the RNA: The RNA was resuspended in 20 ul RNAse-free water, following flicking it genteelly they were transferred to 50°C heat block and centrifuged for 8 minutes. Finally, the pellet was air dried at room temperature for a few minutes, then warped with a plastic paraffin film to avoid contamination and stored at -80 °C.
Figure 6: NanoDrop™ Spectrophotometer analysis: 1 μl of RNA diluted with 19 μl RNase-free water (1:20 dilution); before starting the actual measurement, it blanked with one μl water. Then, by using a 10 μl microcuvette to measure 1 OD of each sample at 260 nm and 280 nm to determine the concentration and the RNA intact and between 1.8 to 2 of the A260/A280 ratio emphasises RNA intact.
Primer design
The primer was designed on the NBCI website by BLAST, which searches the nucleotide sequence and the primer itself. The primer sequence was: Bcl-2: 5′-CGA CTT TGC AGA GAT GTC CA-3′ (forward), 5′-ATG CCG GTT CAG GTA CTC AG-3′ (reverse).
Complementary DNA (cDNA) synthesis: Firstly, a total volume of 12 μl of DNase-free water and RNA was aliquoted into the 12 tubes and heated at 60 °C for 5 minutes. The tubes were placed on ice, then post-spin and placed on ice again.
Table 1, Reagents and their concentration required for cDNA synthesis.
| Components | Volume (μl) |
| 10mM dNTP mix | 2 μl |
| 5X Reaction Buffer | 4 μl |
| RiboLock RNase inhibitor (20U/ μl) | 1 μl |
| RevertAid H minus M-MuLV Reverse Transcriptase (200U/ μl) | 1 μl |
The above components were mixed gently and centrifuged to collect the contents and were incubated for 50 minutes at 50°C; then, the reaction was terminated after increasing the temperature up to 70°C for 5 minutes. The reverse transcription product (cDNA) that could have been used immediately in the PCR was, however, stored at -70°C before the PCR cycle.
Primer Dilution and PCR setup
The BCL-2 forward and reverse primer arrived as a dry pellet at 26.1 nm and was mixed with 260 μl water and vortexed to gain the 100 M to make the working stock solution. Then, 5 M was taken from the 100 M through 1:20 dilution to produce the 1.5 μM final working concentration for each PCR tube.
- Water 105 μl
- HS antibody Tag red 150 μl
- Primer mix 30 μl
These master mixtures for 15 tubes were vortexed and then aliquoted 18 μl into the 13 PCR tubes (triplicated: control, DOX. DOX + MEL and MEL tubes) and into a black control tube. Then 1μl of cDNA was added to the triplicated tubes and was flicked with a finger to mix it.
Table 2, Content of each tube in the PCR tubes.
| Components | Control | DOX | DOX and MEL | MEL M | blank control |
| 2x, MyTaq HS Red Mix, | 10 | 10 | 10 | 10 | 10 |
| Primer mix 1 μl final concentration | 1 | 1 | 1 | 1 | 1 |
| dH2O | 7 | 7 | 7 | 7 | 7 |
| cDNA template 1μl | 1 | 1 | 1 | 1 | No cDNA |
The H9C2 cardiomyocyte cell line was treated with the final concentration of 0.5 μM of Dox and one μM of Melatonin 24 hours before the PCR setup. All the tubes were coated tightly and placed into the PCR apparatus. Then the PCR was performed with the following cycles: 94°C for 5 min, followed by 65°C for 5 minutes, 42 °C for 1 hrs and with 28 cycles annealing at 58°C for 30 s and extension at 72°C for 45 s.
Agarose gel preparation starts with 1.5 g of agarose powder mixed with 100 mL 1x Tris Borate EDTA (TBE) to make 75 gel of the 150 ml. Then to fully dissolve the agarose the mixture was microwaved at maximum power for 2min and kept for it for about 5 minutes at room temperature to cool down the agarose solution, then1μl Gel-Red added swirling it carefully to mix it. The agarose solution was then poured carefully into a gel plate and left for another 25 minutes to set the agarose completely. The agarose gel was transferred to the electrophoresis unit, and then 1xTBE was poured to cover the gel.
Loading samples into the gel: Into the first lane of the gel, cautiously loaded a 100 bp molecular weight ladder, and then from the 20 μl of master mix, 15 μl loaded from samples 1 to 12, a gap then loaded sample 13 negative control with no cDNA.
Running electrophoresis: The PCR products were then separated by running the gel for 15 minutes at 150 V. The current was switched off when the electrophoresis has completed, following separating the electrodes from the power the gel gently removed from the gel box. Then, it was stained with a red gel to finally visualise the DNA fragments using a UV transilluminator at 302 nm.
Data analysis: the BCL-2 gene expression was Sami-quantified using the Java image software. Results were analysed using Prism software, the categorical data are presented as percentage while the normally distribution continuous variables is presented as mean ± SEM. Similarly, differences between the treatment groups were evaluated by an unpaired 2-tailed t-test.
Results
Table 4: Total RNA quantification using NanoDrop 1000 spectrophotometer at A260/A280 ratios. Cardiomyocytes were treated in a 2x six-well plate with or without DOX and MEL for 24 hrs. The cells pre-treated with medium for 2 hours were refiled with DOX + MEL and incubated for 24 hrs. 1ml was taken from each sample: Control, DOX, DOX + MEL and MEL and the absorbance at 260nm measured the total nucleic acid present, and the absorbance at 280nm wavelength estimated the protein level in each sample. Standard requirements for A260/A280 ratios are ~ 1.8 for pure DNA and ~ 2.2 for pure RNA.
| Sample | RNA (2 μl) | Concentration
( ng/ μl ) |
260/280 ratio | RNA per well (μg 20 μl) | μl (1 μg) | Water (μl) |
| 1 | Control | 267 | 1.94 | 5.35 | 3.74 | 8.26 |
| 2 | Control | 283 | 1.95 | 5.60 | 3.53 | 8.47 |
| 3 | Control | 251 | 1.96 | 5.03 | 4.0 | 8.0 |
| 4 | DOX | 196 | 1.88 | 3.93 | 5.1 | 6.9 |
| 5 | DOX | 231 | 1.85 | 4.63 | 4.3 | 7.7 |
| 6 | DOX | 120 | 1.82 | 2.41 | 8.3 | 3.7 |
| 7 | DOX + MEL | 128 | 1.83 | 2.50 | 7.8 | 4.2 |
| 8 | DOX + MEL | 187 | 1.88 | 3.74 | 5.3 | 6.7 |
| 9 | DOX + MEL | 156 | 1.84 | 3.12 | 6.4 | 5.6 |
| 10 | MEL | 190 | 1.90 | 3.81 | 5.3 | 6.7 |
| 11 | MEL | 265 | 1.92 | 5.31 | 3.8 | 8.2 |
| 12 | MEL | 268 | 1.93 | 5.35 | 3.7 | 8.3 |
All samples are presented with the standard required A260/A280 ratio with means: control = ~1.94, DOX = ~ 1.85, DOX + MEL= 1.85 and MEL= ~ 1.92, indicated pure RNA.
Melatonin attenuates DOX-induced down-regulation of BCL-2 expression
to determine whether MEL attenuates DOX-induced apoptosis through the BCL-2 and BCL-XL dependent pathway, a triplicated independent trial was conducted in H9C2 cardiomyocytes. ( Figure 10A) The expression of individual markers of each sample was examined in ImageJ software reading and gathered for further statistical analysis Appendix 1. (F11 C)(Figure bcl2)0.5 µM DOX-treatment for 24 hrs reduced the cell viability to 10 %. Similarly, in the plasma of patients undergoing DOX- DOX-chemotherapy, this specific level was clinically significant to the presence of DOX dose (NefrinA ). H9c2 cells treated for 24 hrs with one µM of MEL alone provided similar cell viability maintenance comparable to control ones. Pre-treated with medium for 2hrs followed with 0.5 µM DOX + 1 µM MEL treatment for 24hrs relatively displayed proliferative influence exhibiting 40 % cardiomyocyte death compared to the controls.
Figure 11.
Control DOX + MEL DOX alone MEL alone.
The effect of MEL and DOX-treatment on H9c2 cardiomyocyte cell sustainability through BCL-2 expression. DOX pre-treatment for 24 hours down-regulates MEL and partially blocks DOX-induced cardiomyocyte cell death by up-regulating BCL-2 expression in the H9C2 cells. The results presented are mean ± SEM from three individual tests performed in triplicate for expression examination of the BCL-XL gene. p = 0.0009 DOX alone significantly different from control, P = 0.001 significantly different from DOX + MEL treated cells, MEL alone P = 0.2 not significantly different from control and p = 0.005 significantly different from control (unrelated 2-tailed test).
Validated normalization is essential to obtain reliable qPCR data
Melatonin up-regulates BCL-XL expression during DOX exposure in H9C2 cells
BCL-XL is a crucial anti-apoptotic factor deactivated in response to various stress factors, including DOX. Down-regulation in BXL-XL can trigger apoptosis. Three independent tests were performed in triplicate to establish the effect of MEL in diminishing DOX-induced cell death by up-regulating the activity of BCL-XL in H9C2 cells. (Figure 10B) The expression of individual markers in each sample examined in Appendix 1. (Figure BXL-XL )0.5 µM DOX-treatment for 24 hrs, one µM MEL alone treatment showed no significant change in the expression levels of BCL-XL compared to control-treated H92C cells. Pre-treated with medium for 2hrs followed by 0.5 µM DOX 1 µM MEL incubation for 24 hrs exhibited a significant increase in the expression of BCL-XL by 50% compared to control groups.
Figure 10. BCL-xl expression level after DOX treatment in the presence or absence of MEL in the H9C2 cells. 1 µM MEL treatment for 24 hours significantly increased cell survival during DOX-induced stress by up-regulating the expression level of BCL-2 expression in the H9C2 cells. The values are presented as mean ± SEM from three individual tests performed in triplicate to examine the expression of the BCL-XL gene. DOX alone group is significantly different compared to control P = 0.007, significantly different compared to DOX alone and MEL P = 0.02. There is a significant difference between the DOX alone and MEL alone groups, p = 0.8 (unrelated 2-tailed test).
Discussion
DOX is one of the most potent antineoplastic drugs used for the treatment of various cancers; however, it is limited due to multiple adverse effects in notable cardiotoxicity (Giuseppe, 2016). Cardiomyocyte cell death via the intrinsic mitochondrial pathway involving disruption of the BCL-2 family protein is a major contributor to DOX-induced cardiomyopathy or heart failure (yu.4). Although numerous studies have been conducted on the protective character of MEL in DIC, these findings generally fail to provide the exact mechanism in which MEL releases its protective effects. The aim of the present study was to establish the influence of MEL against DOX-induced cell death through pathways involving BCL-2 and BCL-XL genes. This investigation hypothesised that DOX would attenuate BCL-2 gene expression, and MEL could repress that effect, thereby enhancing the survival of cardiomyocytes against DIC.
In this project in-vitro cell line model system was conducted to investigate the effect of MEL on BCL-2 and BCL-XL expression during DIC in the H9C2 cell cardiomyocyte, and the main results are as follows: (I) DOX-induced apoptosis via immensely downregulating the anti-apoptotic BCL-2 gene expression (II) pre-incubation with MEL for 24hrs had partially prevented DOX-induced apoptosis via moderately upregulating BCL-2 gene expression. (III) Similarly, pre-treatment with MEL effectively alleviates DOX-induced apoptosis, improving cell survival by enhancing the level of anti-apoptotic BCL-XL pathway molecule in the H9c2 cardiomyocytes (Figures bcl-2&bcl-xl).
Many studies have proposed that apoptosis has a crucial role in the route in the development and progression of DIC. Cardiomyocyte apoptotic cell death initiates throughout different physiological or pathological stimuli, which are monitored via signalling proteins, mainly BCL-2 family genes. The intrinsic apoptosis is dependent on the mitochondrial pathway, which is the leading cause of cardiomyocyte damage and cardiac dysfunction in DIC (yu.4 & gh 6). The anti-apoptotic proteins, including BCL-2, BCL-XL are significantly down-regulated and simultaneously, the pro-apoptotic factors, such as Bax, cytochrome c, caspase-9 and caspase-3, are up-regulated during DOX exposure thereby promoting cardiomyocyte apoptosis. This is in justification with this current result that demonstrated a significant down-regulation of the anti-apoptotic Bcl-2 expression in the H9C2 cardiomyocytes DOX exposed, indicating apoptotic cell deaths (figure bcl-2).
Similarly, (NE,1) established DOX-treated H9c2 cells displayed a significantly decreased expression in the anti-apoptotic protein, Bcl-2, with simultaneous induction of cytochrome c release, increased Bax expression, activated caspase-9 and caspase-3. This finding highlights the crucial function of BCL-2 in the DOX-mediated intrinsic mitochondrial pathway of apoptosis in cardiomyocytes.
Melatonin attenuated DOX-induced apoptosis.
MEL is a direct and indirect cardioprotective antioxidant and anti-apoptotic that appears effective via its radical scavenger and antioxidant enzyme activator during DIC. The anti‐apoptotic properties of MEL are convoyed by its radical-scavenging capability through the alteration of Bcl‐2 family genes. This exhibits upregulation of anti-apoptosis BCL-2 and/or inhibition of the pro-apoptotic related factors such as Bax. This project discovered the positive influence of MEL via its free radical scavenging ability during DIC as it has suppressed DOX enhancing over one-fold of the pro-survival BCL-2 gene expression in the H9C2 cells. To validate this outcome (Yang et al. 2016 Sun et al., 2016) found that H9C2 cells pre-treated with MEL displayed upregulated Bcl-2 and Notch1 gene expressions with downregulated in pro-apoptotic Bax and caspase- 3. This suggests that through the Bcl-2 induction, MEL has abolished Bax and cytochrome c release from the mitochondria, thus preventing the activation of key downstream effector caspase-3 from initiating apoptotic cell death via poly(ADP-ribose) polymerase (PARP). Due to the fact that a declined ratio of Bcl-2/Bax can result in the formation of pores in the mitochondria, and this, in turn, initiates the activation of the apoptotic mechanisms, it is probable that MEL presents defences against Dox-induced cardiomyocyte apoptosis by enhancing the ratio of Bcl-2/Bax.
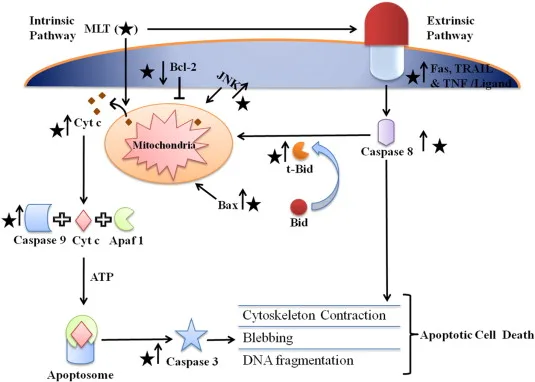
Figure 2: Intrinsic (A) and extrinsic (B) pathways: A, MEL blocks JNK, BAX, BID upregulating anti-apoptotic BCL-2 to inhibit cytochrome c release, in turn, inactivate caspase -9 and caspase -3. B, MEL inhibits Fas, TRAIL and TNF receptors from activating caspase-8 and downregulates t-Bid. Both pathways prevent cytoskeleton contraction, blebbing and DNA fragmentation to avert apoptotic cell death.
Remarkably In this present finding MEL or DOX alone showed no obvious alteration on anti-apoptosis BCL-XL expression, however, under stress condition MEL + DOX significantly inhibited cardiomyocytes apoptosis via heightening the level of pro-survival BCL-Xl. Therefore, it’s possible that MEL excretes its antioxidant protective ability in a stress environment such as DIC. This finding is supported by colleagues, MEL alone treated H9C2 cells showed no alteration in SIRT2 and SIRT3, whilst MEL + DOX significantly blocked DOX-induced inhibition of both genes that are involved in regulating the apoptosis pathways including BCL-2 and BXL-XL genes. Furthermore, pre-treatment with MLT restored SIRT1 and SIRT3 expression in DOX-exposed H9c2 cells, and uncommonly, MLT alone decreased the SIRT1 expression. This corroborates with the current result, which demonstrates that it is probable that MLT releases its protective effect in intense surroundings. Similarly, in the in-vivo model, SIRT1 inhibited the expression of pro-apoptotic proteins Bax, cleaved caspase-3 and increased the expression of Bcl-xL (Liu et al., 2016; Hsu et al., 2010). Suggesting that increasing BCL-XL/ BAX ratios through activation of SIRT1 stimulates cardiomyocyte survival during cardiac failure.
In mechanisms through which melatonin reduces Bax concentrations are unknown, Bcl‐2 up‐regulation possibly appears via the activation of an inner mitochondrial membrane uncoupling protein 2 (UCP2) that plays a crucial role in reducing oxidative stress-induced cell deaths. In an H9C2 cell, Dox-induced apoptosis, oxidative stress and decline in UCP2 and Bcl-2 levels along with an increase in Bax level through the eIF2α-CHOP-dependent mitochondrial pathway and these effects were blocked by MEL (UB31). Also, MEL alone has been shown to improve UCP2, which is similar to this current outcome, as MEL alone has demonstrated a slight increase in BCL-2 expression. Cooperatively, this data provides a solid indication that BCL-2 and UCP-2 have a distinct anti-apoptotic effect on MEL-mediated oxidative stress decline and cardiomyocyte apoptosis. These suggested that a method to activate BCL-2 and UCP-2 in cardiomyocytes could deliver a novel route to diminish DIC.
Melatonin on DOX-induced autophagy
Apoptotic and autophagy pathways are closely related to different interactions involving BCL-2, a negative effector of the autophagy mediator Beclin1 in the mitochondrial. This study demonstrated that DOX-treated cardiomyocytes displaying a significant reduction in the BCL-2 expression are possible under overwhelmed autophagy. In support of this, co-incubated with cardioprotective agent acetaminophen diminished autophagy beside cardiotoxicity after DOX treatment by activating the Bcl-2 transcriptional inducer GATA4 pathway in H9C2 rat cardiomyoblasts and neonatal rat cardiomyocytes (S.3). However, the above-presented discovers had challenged by the observation in H9C2 cardiomyoblasts, were stimuli activating autophagy such as mTOR inhibition or AMPK activation resulted in improved cell sustainability and attenuated apoptosis following DOX treatments. Nevertheless, one of the critical considerations made by this study was that DOX stimulates cardiotoxicity by increasing autophagy and that inhibiting autophagy by MEL-mediated Bcl-2 and BCL-XL activation inverts cardiotoxic outcomes. MEL mediates its cardioprotective properties by deactivating autophagy, and hence, downregulating autophagy may be used to decrease the harmful outcomes of DOX-related diseases.
Thinking to include Innovative mechanism
MEL
UCP-2 BCL-2 /Bax cardiomyocyte survival
SIRT 3
PGC1 Beclin-1 declines autophagy inhibition
Figure …
Conclusion
In this current study, an H9C2 Cell model system was conducted to investigate the protection of MEL via anti-apoptotic pathways during DIC. Together, the results demonstrate that DOX-induced apoptosis through BCL-2 reduction and MEL moderately restored this, and under stress conditions, MLT significantly inhibited apoptosis by substantially increasing the sustainability of BCL-XL in DIC. Similarly, MEL moderately inhibits the DOX effect on the BCL-2 activity, while treatment with MEL alone displayed no alteration in both critical apoptotic inhibitor agents BCL-2 and BCL-XL. Typically, an increase in the anti-apoptotic level is accompanied by decreased pro-apoptotic levels presented as an increased BCL-2/BAX ratio, which prevents cardiomyocyte apoptosis.
As cell death is central to the clinical limitation of DOX, the underlying mechanisms involved in the anti-apoptotic effects of MLT through the upregulation of anti-apoptotic including BCL-2 and BCL-XL. Similarly, a reduction in the BCL-2, a negative modulator of Beclin1, leads to autophagic dysregulation during DIC. Therefore, it’s worth exploring the signalling of both BCL-2 and BCL-XL that are responsible for inhibiting the pro-apoptotic downstream cascades BAX, cytochrome c, caspase-9 and death effector caspase-3 since MLT could hypothetically operate as a cardioprotective mediator in the perspective of DIC. This is in validation with this existing outcome with a significant down-regulation of Bcl-2 activity during DIC and BCL-XL and BCL-2 upregulation with DOX + MEL treatments (figure both)
Further Experiments
This current experiment established an H9C2 cell model system to investigate drug-induced cardiotoxicity and anti-apoptotic protective agents through BCL-2 and BCL-XL pathways. The analysis used a single concentration and duration, which was 0.5 uM of DOX and 1 uM of MEL, with 24-hour incubation, and the DOX + MEL treatment was applied at the same time. The adaptive response gathered from this study depends on the H9c2 cell model and the genes used, on the DOX stimuli, duration of injury together, and the experimental conditions. However, in a further experiment, these parameters could be changed to improve the outcomes.
In the future, the experiment should also be replicated in a primary cell or can be used in a place of the H9C2 cell line because the cell line often does not adequately reproduce primary cells and could deliver different results. Primary cells have normal cell morphology and maintain many of the critical markers and functions seen in vivo. Even though working with primary cells may be more challenging, the results collected from primary cells are more relevant and reflective of in vivo models.
Moreover, the MEL protective role may be enhanced by using a variety of concentrations such as MEL treatment with 2, 4, 6 μM and 4, 8, and 12 hrs duration. Also, the H9C2 cells could be treated with DOX acutely, then with MEL chronically or pre-incubated 1.5 μM of MEL for 24hrs then added one μM of DOX with a 12-hour incubation may increase BCL-2 expression. A study conducted in H9c2 cells in-vitro model revealed that pre-treated with MLT for 24 hrs improved cell survival following DXR treatment at three μM for 24 hrs. It is possible that longer exposure time to MLT could increase the cells’ antioxidant capacity.
Furthermore, DOX is reported to elevate intracellular hydrogen peroxide followed by mitochondrial cytochrome c release and apoptosis mediator caspase-3 activation accompanied by up-regulation of pro-apoptotic protein and down-regulation of anti-apoptotic protein. Therefore, hydrogen peroxide could be used as a stress inducer in place of DOX to induce cardiomyocyte apoptosis. Additionally, since cell death is central to the clinical limitation of DOX, the underlying mechanisms involving pro-apoptotic downstream cascades BAX, cytochrome c, caspase-9 death effector caspase-3 are worth investigating.
Cite This Work
To export a reference to this article please select a referencing stye below: